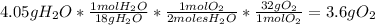Answer:
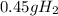
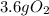
Step-by-step explanation:
The problem gives you the balanced reaction:
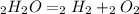
To calculate the mass formed of each product you need to have the molar mass of reactant and products, so:
molar mass of
 =
=
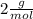
molar mass of
 =
=
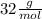
molar mass of
 =
=
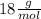
Then you should use the stoichiometry to make the relationships between the moles of products and the reactant:
- For
 :
:
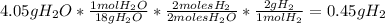
-For
 :
: