Step-by-step explanation:
- It is known that the amount of heat necessary to raise the temperature of 1 gram of a substance by
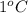 is known as specific heat.
is known as specific heat.
Since, q =
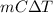
So, larger is the specific heat of a substance less will be the change in its temperature.
Therefore, olive oil has less specific heat as compared to water. This means that olive oil would get hotter.
- Similarly, the specific heat of gold is lesser than the given materials or metals. Hence, gold will requires less heat to rise its temperature.
As a result, water present in gold will heat readily.
- As the relation between heat and specific heat is as follows.
q =
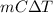
Therefore, calculate the amount of heat required by the water as follows.
q =
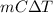
=
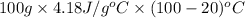
= 33440 J
or, = 33.44 kJ (as 1 kJ = 1000 J)
Thus, 33.44 kJ heat would it take to raise the temperature of 100.0 g of water from
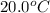 to
to
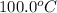 .
.