Answer:
trans-1,3-pentadiene is more stable than 1,4-pentadiene due to presence of a conjugated double bond.
Step-by-step explanation:
Here,
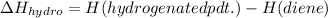
H(hydrogenated pdt.) is same for both 1,4-pentadiene and 1,3-pentadiene as they both produce pentane after hydrogenation
H(diene) depends on stability of diene.
More stable a diene, lesser will be it's H(diene) value (more neagtive).
trans-1,3-pentadiene is more stable than 1,4-pentadiene due to presence of a conjugated double bond.
Hence,
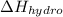 is higher (less negative) for trans-1,3-pentadiene
is higher (less negative) for trans-1,3-pentadiene