Step-by-step explanation:
Kinetic energy is the energy obtained by an object because of its motion.
And, kinetic energy is directly related to temperature as follows.
K.E =
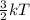
where, k = Boltzmann constant
T = temperature
As temperature is also a measure of average kinetic energy of particles. Therefore, when temperature is decreased then there will also occur a decrease in the kinetic energy of particles.
This will lead to change in phase of the substance as decrease in kinetic energy of particles of a liquid will change it into solid phase. And, an increase in kinetic energy of particles of a liquid will lead to change it into vapor state.