Answer:
In this conditions, the gaswll weight 46.74 g.
Step-by-step explanation:
The idal gas law states that:
PV = nRT,
P: pressure = 740 mmHg = 0.97 atm
V: volume = 14.5 L
n: number of moles
R: gas constant =0.08205 L.atm/mol.K
T: temperature = 29°C = 302.15K
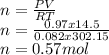
1 mol gas ___ 82 g
0.57 mol gas __ x
x = 46.74 g