Answer: The molar concentration of ethylenediminediacetic-dihydrate and sodium ions in solution is 0.1976 M and 0.3952 M respectively.
Step-by-step explanation:
To calculate the molarity of solution, we use the equation:
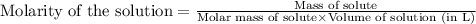
We are given:
Mass of solute (disodium ethylenediaminetetraacetic acid dihydate) = 36.7845 g
Molar mass of disodium ethylenediaminetetraacetic acid dihydate = 372.24 g/mol
Volume of solution = 0.5000 L
Putting values in above equation, we get:
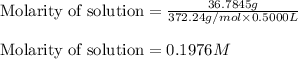
As, 1 mole of disodium ethylenediaminetetraacetic acid dihydate produces 2 moles of sodium ion and 1 mole of ethylenediminediacetic-dihydrate.
Concentration of ethylenediminediacetic-dihydrate in solution =
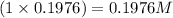
Concentration of sodium ions in solution =
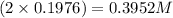
Hence, the molar concentration of ethylenediminediacetic-dihydrate and sodium ions in solution is 0.1976 M and 0.3952 M respectively.