Answer:
To prepare 100 mL of a 0,050M sodium citrate tribasic buffer to a pH of 6 you need to add 7,16 mL of 0,5M HCl, 1,4705 g of sodium citrate tribasic dihydrate and complete 100 mL with water.
Step-by-step explanation:
The acid equilibrium of sodium citrate tribasic buffer is:
citrate dibasic⁻² ⇄ citrate tribasic⁻³ + H⁺ pka = 6,4
Using Henderson-Hasselbalch formula:
pH = pka + log₁₀
![([A^(-)])/([HA])](https://img.qammunity.org/2020/formulas/chemistry/college/aqob76gnk5at0cs1bms0w0c2gm6z8849is.png)
6,0 = 6,4 + log₁₀
![([CitrateTribasic])/([CitrateDibasic])](https://img.qammunity.org/2020/formulas/chemistry/college/mc2u1yjdi30jal42od923wg7nalxxj9uy6.png)
0,3981 =
![([CitrateTribasic])/([CitrateDibasic])](https://img.qammunity.org/2020/formulas/chemistry/college/mc2u1yjdi30jal42od923wg7nalxxj9uy6.png) (1)
(1)
You need to add 0,1L× 0,050M = 0,0050moles of sodium citrate:
0,0050 moles = Citrate tribasic + Citrate dibasic (2)
Replacing (2) in (1)
Citrate dibasic: 3,58x10⁻³ moles
Thus,
Citrate tribasic: 1,42x10⁻³ moles
Citrate tribasics reacts with HCl thus:
Citrate tribasic⁻³ + HCl → Citrate dibasic⁻² + Cl⁻
Thus, you need to add 5x10⁻³moles of sodium citrate tribasic and 3,58x10⁻³ moles of HCl:
5x10⁻³moles of sodium citrate tribasic×
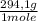 = 1,4705 g of sodium citrate tribasic dihydrate -commercial reactant-
= 1,4705 g of sodium citrate tribasic dihydrate -commercial reactant-
3,58x10⁻³ moles of HCl÷ 0,5 M = 7,16x10⁻³ L ≡ 7,16 mL of 0,5M HCl
Thus, to prepare 100 mL of a 0,050M sodium citrate tribasic buffer to a pH of 6 you need to add 7,16 mL of 0,5M HCl, 1,4705 g of sodium citrate tribasic dihydrate and complete 100 mL with water.