Answer : The structure of iso-propyl and iso-butyl alkyl groups are shown below.
Explanation :
Hydrocarbon : It is an organic compound that contains only hydrogen and the carbon atoms.
The propyl group is the hydrocarbon part of the of the monosubstituted alkane. The formula of propyl group is
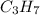 . There are two types of propyl group that are n-propyl and iso-propyl.
. There are two types of propyl group that are n-propyl and iso-propyl.
The n-propyl group is formed by removing the hydrogen atom from one of the terminal carbon atom.
The iso-propyl group is formed by removing the hydrogen atom from the middle (second carbon) of carbon atom.
The butyl group is the hydrocarbon part of the of the monosubstituted alkane. The formula of butyl group is
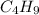 . The iso-butyl group is formed by removing the hydrogen atom from the middle (second carbon) of carbon atom.
. The iso-butyl group is formed by removing the hydrogen atom from the middle (second carbon) of carbon atom.
The structure of iso-propyl and iso-butyl alkyl groups are shown below.