Answer:
The volume of seawater needed to extract
 tons of magnesium is
tons of magnesium is
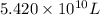 .
.
Step-by-step explanation:
Concentration magnesium in sea water = 1.3 g /kg of seawater
Extracted mass of magnesium ,=
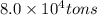
(1 ton = 907185 g)
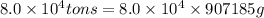
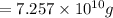
If 1 kg of sea water contains 1.3 grams of magnesium. Then
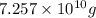 of magnesium will be contained by:
of magnesium will be contained by:
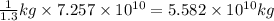 sea water.
sea water.
Mass of sea water,m =
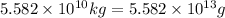
Volume of sea water = v
Density of sea water ,d= 1.03 g/mL
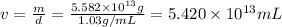
1 mL = 0.001 L
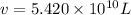
The volume of seawater needed to extract
 tons of magnesium is
tons of magnesium is
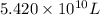 .
.