Answer:
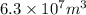
Step-by-step explanation:
Required amount of magnesium =
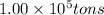
Given : 1 ton = 2000 lb
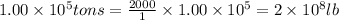
1 lb = 453.592 g

Given : 1.4 g of magnesium is produced by 1000 g of sea water
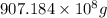 of magnesium is produced by =
of magnesium is produced by =
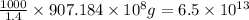 g of sea water
g of sea water
Density of sea water = 1.025 g/ml
Volume of sea water =
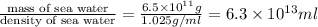
1 ml =
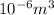
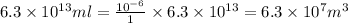
Volume of seawater, in cubic meters is
