Answer:
2835 kilograms of sodium hypochlorite must be added to the water supply each week to produce the required chlorine level of 1 ppm.
Step-by-step explanation:
Volume of water used by 1 person = 750 L
Volume of water used by 1.8 million persons : V
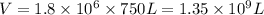
Density of water,d = 1 kg/L
Mass of water used by 1.8 million persons = m
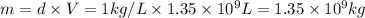
1 kilogram of chlorine per million kilograms of water. (Given)
Concentration of chlorine in water = 1 kg/ 1000,000 kg of water
In 1000,000 kg of water = 1 kg of chlorine
Then
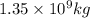 of water have x mass of chlorine:
of water have x mass of chlorine:
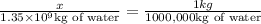
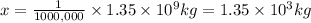
Mass of chlorine in water of mass
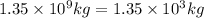
Percentage of chlorine in hypochlorite = 47.62%
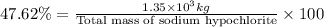
Total mass of sodium hypochlorite =
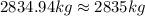
2835 kilograms of sodium hypochlorite must be added to the water supply each week to produce the required chlorine level of 1 ppm.