Answer:
2.5 grams of of water is consumed by the reaction of 6.2 g of carbon dioxide.
Step-by-step explanation:
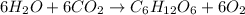
Moles of carbon dioxide =
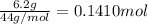
According to reaction , 6 moles of carbon dioxide reacts with 6 moles of water.
Then 0.1410 moles of carbon dioxide will react with:
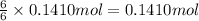 of water.
of water.
Mass of 0.14109 moles of water = 0.1410 mol × 18 g/mol = 2.536 g ≈ 2.5 g
2.5 grams of of water is consumed by the reaction of 6.2 g of carbon dioxide.