Answer:
25 milliliters
Explanation:
Given:
volume of sample = 100 ml
Syrup present in sample = 6.55 g per 20 mL
Therefore in 100 mL, amount of syrup present =

or
amount of syrup present in 100 mL sample = 32.75 grams
Now,
Specific gravity of the syrup = 1.31
also,
Specific gravity of the syrup =
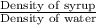
or
1.31 =
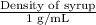
or
Density of syrup = 1.31 g/mL
also,
Density =
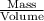
therefore,
1.31 =

or
Volume of syrup in the sample =
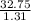
or
Volume of syrup in the sample = 25 milliliters