Answer:
The percentage error in the measurement is 2.4%
Step-by-step explanation:
The measurement made by the student is
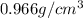 .
.
But the actual value of density is
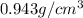
The formula to calculate percentage error is
percentage error =
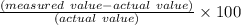
Hence percentage error in this case is given by
Percentage error =
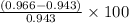
=
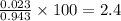
A physical quantities value will be determines by various quantities. If errors are made in measuring any of these quantities there will be error in the measurement of the final physical quantity as well.
Here density of a substance is a derived quantity determined by measuring mass and volume of that substance. If errors are made in mass and volume measurements the density value will also be erroneous.