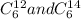Answer:
Atomic number and protons
The possible difference may be in number of neutrons.
Step-by-step explanation:
1) The elements are characterized by their atomic number.
The atomic number is the number of protons in an atom of an element.
So atoms of same elements will have same number of protons.
The number of neutrons may vary in atoms of same element if they are different isotopes.
Electron number in neutral atoms will be same, however in case of gain or loss of electrons the number may change.
So the atomic number and protons must be same.
2) Isotopes are substances which have same atomic number but different mass number (due to difference in number of neutrons).
So there is possibility that if we are taking atom of carbon from a rock and from skin cell, they are isotopes.
The following are the common natural isotopes of carbon