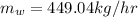Answer:
Step-by-step explanation:
Given data:
air stream temp 32.2 degree celcius
steam heater is at 65.5 degree celcius
flow of air 1000 kg mol/h
we know that
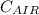 = 29.2 Kj/KG-MOL
= 29.2 Kj/KG-MOL
Saturated stream is at 148.9 and from steam table hg is 2745.12 kJ/kg
saturated water us 148.9 degree and from steam table hf = 627.46 kJ/kg
then cooled to saturated water with temperature 137.8 degree celcius and hf = 579.71 kJ/kg
from energy balanced
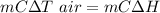
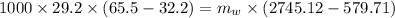
solving for m_w