Answer:
Theoretical yield is 0,52 g and percent yield for this reaction is 73%
Step-by-step explanation:
The theoretical yield is the maximum amount of product a chemical reaction could create based on chemical equations. The percent yield is the ratio between theoretical yield and obtained yield times 100.
In the reaction:
2-butanone + propyl magnesium bromide → 3-methyl-3-hexanol
The theoretical yield with 0,40 mL is:
0,40 mL×
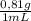 ×
×
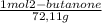 ×
×
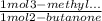 ×
×
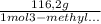 = 0,52 g
= 0,52 g
Thus, percent yield for this reaction is:
0,38g/0,52g×100 = 73%
I hope it helps!