Answer:
Step-by-step explanation:
In English;
If a few drops of acid are introduced into a shell, the effervescence may be observed. The shell contains calcium carbonate.If 1.46g of hydrochloric acid have been introduced, what quantities of reaction products are obtained?
Given parameters:
Mass of HCl = 1.46g
Unknown:
Mass of CO₂ produced = ?
Mass of H₂O produced =?
Mass of CaCl₂ produced = ?
Solution
The balanced reaction equation is given below:
CaCO₃ + 2HCl → CaCl₂ + CO₂ + H₂O
The effervescence observed is due to the production of carbon dioxide gas.
In order to find the mass of each of the products obtain, we must first express the known mass of the acid in terms of moles:
number of moles of HCl =
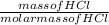
Molar mass of HCl = 1 + 35.5 = 36.5g/mol
number of moles of HCl =
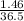 = 0.04mole
= 0.04mole
Now, we must find the number of moles of each of the products obtained
number of moles of CaCl₂:
2 moles of HCl produced 1 mole of CaCl₂
0.04 moles of HCl will produce
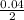 moles = 0.02moles of CaCl₂
moles = 0.02moles of CaCl₂
Also, number of mole of H₂O is 0.02moles
number of mole of CO₂ is 0.02moles
Mass of the products:
Mass of CaCl₂ = number of moles of CaCl₂ x molar mass of CaCl₂
molar mass of CaCl₂ = 40 + 2(35.5) = 111g/mol
mass of CaCl₂ = 0.02 x 111 = 2.22g
Mass of H₂O = number of moles of H₂O x molar mass of H₂O
molar mass of H₂O = 2(1) + 16= 18g/mol
mass of H₂O = 0.02 x 18 = 0.36g
Mass of CO₂ = number of moles of CO₂ x molar mass of CO₂
molar mass of CO₂ = 12 + 2(16) = 44g/mol
mass of CO₂ = 0.02 x 44 = 0.88g