Answer:
The mass of acrylonitrile is 106 g.
Step-by-step explanation:
The equation is balanced and considering that the yield is 100%, the mass can be calculated using the following formula:
number of mols=
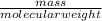
The molecular weight for C3H3N is 53 g/mol. Since the number of mol is 2 according to the equation, the mass will be 106 g.