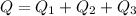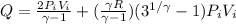Answer:
Step-by-step explanation:
As we know that at isochoric conditions if pressure of the gas is tripled then the temperature also becomes 3 times
So heat given in this process is given as
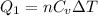
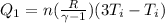
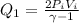
Now it is expanded to initial pressure again by adiabatic process
So in this part there is no heat exchange
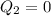
Also we know that
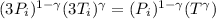
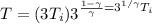
Now in the last process we compressed the gas to original volume
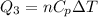
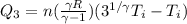
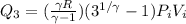
Now total heat in the process is given as