Answer:
The percentage (by mass) of KBr in the original mixture was 33.1%.
Step-by-step explanation:
The mixture of KCl and KBr has a mass of 3.595g, thus the sum of the moles of KCl (x) multiplied by it molar mass (74.5g/mol) and the moles of KBr (y) multiplied by it molar mass (119g/mol) is the total mass of the mixture:
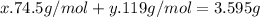
Also, after the conversion of KBr into KCl, the total mass of 3.129 g is only from KCl moles, hence
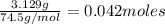
But the 0.042 moles came from the originals KCl and KBr moles, thus
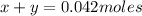
Now it is possible to propose a system of equations:
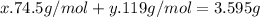
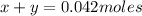
Solving the system of equations,
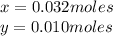
0.010 moles of KBr multiplied it molar mass is
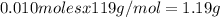
Therefore, the percentage (by mass) of KBr in the original mixture was:
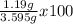 %
%