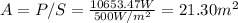Answer:
21.30 m2
Step-by-step explanation:
First of all, we calculate the energy necessary to increase the temperature of the water from 20°C to 49°C. We use the specific heat in order to achieve this:
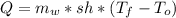
Where m_w is the mass of water, sh is the specific heat.
The mass of the water can be found using the volume of water and density:
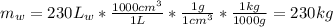
Then:
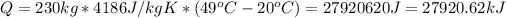
You want to heat the water in 2.6h, this means, you will need to supply a power equal to the total amount of energy required divided by the time in seconds. In 2.6h there are 2.6*3600 = 9360s:
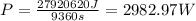
But remember, you have an efficiency of 28%, so the actual amount of power that you need to recieve from the sun is equal to:
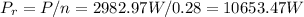
Now, for the power of a collector is:

Where Psun is the power of the sun, S is the intensity of incident sunlight and A is the area. Solving for Area: