Step-by-step explanation:
The given data is as follows.
Energy needed for 1 mole = 279.7 kJ =
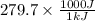
= 279700 J
Therefore, energy required for 1 atom will be calculated as follows.
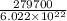
=
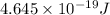
As relation between energy and wavelength is as follows.
E =
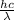
where, h = planck constant =
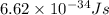
c = speed of light =
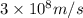
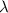 = wavelength
= wavelength
Therefore, putting given values into the above formula as follows.
E =
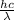
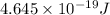 =
=
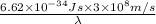
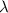 =
=
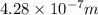
or, =
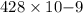 m
m
= 428 nm
Thus, we can conclude that the maximum wavelength of light that can remove an electron from an atom on the surface of lithium metal is 428 nm.