Answer:
A negative ∆H means that energy is given off from a reaction
Step-by-step explanation:
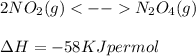
If the reaction has negative ∆H value then the reaction is exothermic
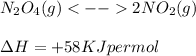
If the reaction has positive ∆H value then the reaction is endothermic
When Heat energy is given in the equation itself
If it is present on the left side it means that heat is absorbed and the reaction is Endothermic
If it is present on the right side it means heat is given off and the reaction is exothermic