Answer:
0.034%
Step-by-step explanation:
First, let's calculated the average density (da) of the two experimental, which the sum of them divided by 2:
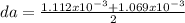
da = 1.090x10⁻³ g/cm³
The relative error is given by the difference of the correct value and the avarege, divided by the correct value:

e = 3.39x10⁻⁴ x 100%
e = 0.034%