Answer:
= - 26.31 kJ
Step-by-step explanation:
we know that number of moles is calculated as
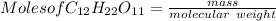
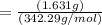
= 0.00476 mol
Heat absorbed by calorimeter
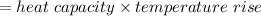
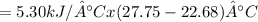
= 26.87 kJ
Enthalpy of combustion
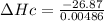
= - 55290.12 kJ/mol
Negative sign shows that the heat is released
The balanced reaction
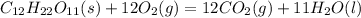
ΔHc = ΔU + Δng (RT)
-55290.12 = ΔU + (12 - 12) *(RT)
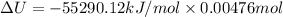
= - 26.31 kJ