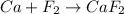Answer:
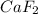 is formed here.
is formed here.
Step-by-step explanation:
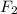 is an oxidizing agent and Ca is a reducing agent because F atom is highly electronegative and Ca atom is highly electropositive.
is an oxidizing agent and Ca is a reducing agent because F atom is highly electronegative and Ca atom is highly electropositive.
Therefore Ca is oxidized to
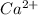 ion by giving electrons to
ion by giving electrons to
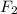 and reduces
and reduces
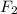 to
to
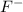 ion.
ion.
Therefore an insoluble ionic compound
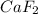 is formed as a result.
is formed as a result.
Balanced equation: