Answer:
8.25 moles of oxygen will be produced.
Step-by-step explanation:
Given a balanced chemical equation;
2Al₂O₃ ------> 4Al + 3O₂
from the balanced chemical equation above, 3 moles of Oxygen are produced when 4 moles of Aluminum are produced.
4 moles of Al --------> 3 moles of Oxygen
11 moles of Al --------> ?
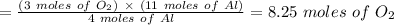
Therefore, 8.25 moles of oxygen will be produced.