Answer:0.0704 kg
Step-by-step explanation:
Given
initial Absolute pressure
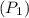 =210+101.325=311.325
=210+101.325=311.325
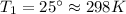
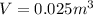
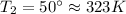
as the volume remains constant therefore
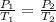
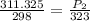
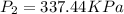
therefore Gauge pressure is 337.44-101.325=236.117 KPa
Initial mass
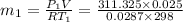
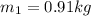
Final mass
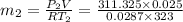
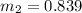
Therefore
 =0.91-0.839=0.0704 kg of air needs to be removed to get initial pressure back
=0.91-0.839=0.0704 kg of air needs to be removed to get initial pressure back