Answer:
Molar percent of sodium in original mixture is 88,50%
Step-by-step explanation:
The last reaction is:
BaCl₂ + Na₂SO₄ → BaSO₄ + 2 NaCl
The moles of BaCl₂ are:
0,132L × 3,80M = 0,502 moles of BaCl₂
As the amount of BaCl₂ is the maximum possible to produce BaSO₄, the moles of BaCl₂ must be the same than moles of Na₂SO₄.
0,502 moles of BaCl₂ ≡ 0,502 moles of Na₂SO₄
These moles of Na₂SO₄ comes from:
2 Na + H₂SO₄ → Na₂SO₄ + H₂
As the reaction is in stoichiometric amounts, moles of Na are twice the moles of Na₂SO₄
0,502 moles of Na₂SO₄ ×
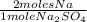 × 22,99 g/mole = 23,08 g of Na
× 22,99 g/mole = 23,08 g of Na
Molar percent of sodium in original mixture is:
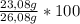 = 88,50%
= 88,50%
I hope it helps