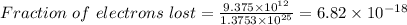Answer:
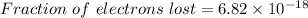
Step-by-step explanation:
Mass of copper = 50 g
Molar mass of copper = 63.5 g/mol
The formula for the calculation of moles is shown below:

Thus,
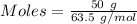
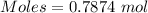
Also,
1 atom of neutral copper contains 29 electrons
1 mole of neutral copper contains
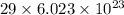 electrons
electrons
0.7874 moles of neutral copper contains
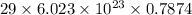 electrons
electrons
0.7874 moles of neutral copper contains
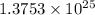 electrons.
electrons.
Given, Charge = 1.5 μC
1 μ = 10⁻⁶ C
So, Charge on the copper =
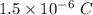
Charge on 1 electron =
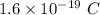
Thus, number of electrons = Total charge / Charge on one electron
Thus,
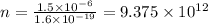
Fraction :