Answer:
zirconium
Step-by-step explanation:
Given, Mass of AgBr(s) = 23.0052 g
Molar mass of AgBr(s) = 187.77 g/mol
The formula for the calculation of moles is shown below:
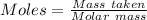
Thus,
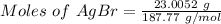
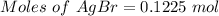
The reaction taking place is:
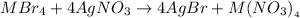
From the reaction,
4 moles of AgBr is produced when 1 mole of
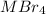 undergoes reaction
undergoes reaction
1 mole of AgBr is produced when 1 / 4 mole of
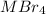 undergoes reaction
undergoes reaction
0.1225 mole of AgBr is produced when
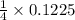 mole of
mole of
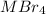 undergoes reaction
undergoes reaction
Moles of
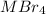 got reacted = 0.030625 moles
got reacted = 0.030625 moles
Mass of the sample taken = 12.5843 g
Let the molar mass of the metal = x g/mol
So, Molar mass of
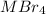 = x + 4 × 79.904 g/mol = 319.616 + x g/mol
= x + 4 × 79.904 g/mol = 319.616 + x g/mol
Thus,
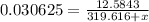
Solve for x,
we get, x = 91.2999 g/mol
The metal shows +4 oxidation state and has mass of 91.2999 g/mol . The metals is zirconium.