Answer:
The volume of 0.244 M KCl solution is required 86.07 mL.
Step-by-step explanation:
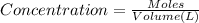
Moles of lead nitrate in 50.0 mL of 0.210 M solution be n.
Molarity of the lead nitrate solution = 0.210 M
Volume of the solution = 50.0 mL = 0.050 L ( 1 mL = 0.001 L)
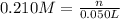
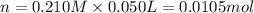
According to reaction given, 1 mole of lead nitrate reacts with 2 moles of KCl.
Then 0.0105 moles of lead nitrate will react with:
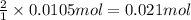 of KCl
of KCl
Moles of KCl = 0.021 mol
Volume of KCl solution = V
Molarity of the KCl solution = 0.244 M
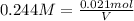
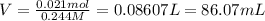
The volume of 0.244 M KCl solution is required 86.07 mL.