Answer:
139.94 grams of solute is present in kilograms of water
Step-by-step explanation:
Temperature of the ice water = T = 1.0°C
Temperature of the mixture =
 =-3.0°C
=-3.0°C
Depression in freezing point
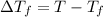
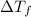 =1.0°C-( -3.0°C)=4°C
=1.0°C-( -3.0°C)=4°C
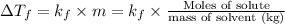
m = molality of the solution
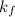 = Molal depression constant
= Molal depression constant
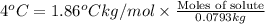
Mole of solute = 0.1705 moles
Mass of solute = 11.1 g
Moles of solute =
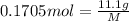
Molar mass of the solute= M
M = 65.088 g/mol
Molality of the solution = m =
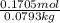
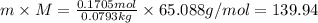 g of solute /kg of water
g of solute /kg of water
139.94 grams of solute is present in kilograms of water