Answer:
The moles of Chlorine in 4.85 g BCl₃ is 0.1242 moles
Solution and Explanation:
- Boron trichloride is an inorganic compound containing one Boron atom and three chlorine atoms.
Step 1: Mass of Chlorine in 4.85 g BCl₃
- 1 mole of BCl₃ contains three atoms of Chlorine
- But, the molar mass of BCl₃ is 117.17 g/mol and the atomic mass of Chlorine is 35.45 g
- Therefore; 117.17 g BCl₃ contains 106.35 g (35.45 g ×3) chlorine.
- Hence; 4.85 g BCl₃ will contain;
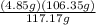
= 4.402 g Chlorine
Step 2: Moles of Chlorine in 4.85 g BCl₃
- Number of moles is given by mass divided by atomic mass
Therefore;
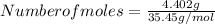
= 0.1242 moles
Hence; 4.85 g BCl₃ contains is 0.1242 moles Chlorine