Step-by-step explanation:
3). A chemical formula is defined as symbolic representation of a molecule or compound.
The number present as a subscript in a chemical formula depicts the number of atoms of each element present in the compound and the ratio in which they are combining.
For example, chemical formula of phosphorous tetraoxide is
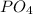 .
.
Therefore, the chemical formula of a compound does NOT indicate how elements are joined in the compound.
4). A chemical reactant is defined as a specie present on the left side of a chemical reaction equation. Whereas a product is a specie that is written on the right side of a chemical reaction equation.
For example,
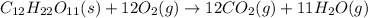
Therefore, in the chemical reaction in which sucrose is heated and decomposes to form carbon dioxide and water, sucrose is a reactant.
5). A change that does not bring any difference in chemical composition of a substance are known as physical change.
For example, shape, size, mass, volume, density, etc of a substance are all physical changes.
And, a change which brings difference in chemical composition of a substance is known as chemical change.
For example, precipitation, reactivity, toxicity etc are chemical changes.
Therefore, fermenting of cheese is a chemical change and NOT a physical change.
6). Decay of wood is a chemical process because in this process fungus or any other specie tends to digest moist wood. As a result, the wood gets rotten and a change in its chemical composition occur.
Therefore, a chemical change occurs when a piece of wood decays.
7). As chemical composition of a substance changes in a chemical change. This may bring changes like formation of a gas, precipitate formation, endothermic or exothermic process etc.
Whereas fracture information is a physical change because it only brings change in shape of the substance.
Hence, fracture formation does NOT indicate that a chemical change may have taken place.
8). According to law of conservation of matter, matter can neither be created nor it can be destroyed. It can only be changed from one form to another.
Therefore, during a chemical reaction matter is neither destroyed or created.