Answer: Option (c) is the correct answer.
Step-by-step explanation:
It is known that a stronger element is able to displace a less stronger element in a chemical reaction.
And, according to the reactivity chart hydrogen is more reactive in nature than copper.
Therefore, when Cu is added to
 then there will be no reaction because copper is less reactive than hydrogen.
then there will be no reaction because copper is less reactive than hydrogen.
Thus, we can conclude that the reaction
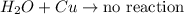 not proceed to a product because copper has a lower activity than hydrogen and cannot replace it.
not proceed to a product because copper has a lower activity than hydrogen and cannot replace it.