Answer:
The energy
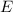 of a photon is given by:
of a photon is given by:
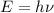 (1)
(1)
Where:
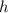 is the Planck constant
is the Planck constant
 is the frequency
is the frequency
On the other hand, there is an inverse relation between the frequency and the wavelength
 :
:
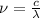 (2)
(2)
Where
 is the speed of light, which is also a constant.
is the speed of light, which is also a constant.
If we substitute (2) in (1) we will have the following:
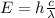 (3)
(3)
This means, there is direct relation between the energy and the frequency and there is an inverse relation between the energy and the wavelength.