Answer:
= 2.2 g pf S. produced
Step-by-step explanation:
Balanced Reaction equation:
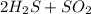 →
→
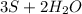
1 mole of H2S - 34.1g
? moles - 3.2g
= 3.2/34.1 = 0.09 moles of H2S
Also,
1 mole of S02 - 64.07 g
? moles - 4.42g
= 4.42/64.07 = 0.069 moles of SO2
Meaning SO2 is the limiting reagent
Finally, 3 moles of S - 32g of sulphur
0.069 mole = ? g of Sulphur
= 0.069 x 32
= 2.2 g pf S.