Answer: This element has 7 protons, 7 electrons and 7 neutrons.
Step-by-step explanation:
There are 3 subatomic particles present in an atom. They are: protons, electrons and neutrons.
- Protons: They carry positive charger and are present in nucleus.
- Electrons: They carry negative charge and are present around the nucleus.
- Neutrons: They dos not carry any charge and are present in nucleus.
Atomic number is defined as the number of protons or electrons that are present in a neutral atom.
Atomic number = number of protons = number of electrons
Mass number is defined as the sum of number of protons and neutrons that are present in an atom.
Mass number = Number of protons + Number of neutrons
Atomic mass is the sum of masses of each isotope each multiplied by their natural fractional abundance. This is also related to protons and neutrons present in an atom.
We are given:
An element having formula
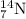
Atomic number = Number of electrons = Number of protons = 7
Mass number = 14
Number of neutrons = 14 - 7 = 7
Atomic mass of nitrogen = 14.01 amu
Hence, the given element has 7 protons, 7 electrons and 7 neutrons.