Answer:
Atomic number = number of electrons = number of protons
We get to know the atomic number of Nitrogen as 7 from the periodic table.
So Nitrogen contains 7 protons and 7 electrons.
To find the number of neutrons we make use of the formula
Mass number - atomic number = number of neutrons
So number of neutrons in a Nitrogen atom =14-7=7 neutrons.
Atomic mass of Nitrogen is 14.0067 amu.
Average Atomic mass is the sum of the atomic mass of the isotopes multiplied by the abundance
Thats why we get a fractional number as atomic mass.
If suppose an element has 3 isotopes then,
Average atomic mass
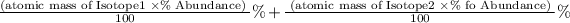
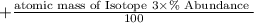
Is the formula to find the average atomic mass of an element.
So the Answer is Nitrogen atom has 7, protons 7, electrons and 7 neutron