Answer: 4. the principal quantum number n.
Step-by-step explanation:
Magnetic Quantum Number : It describes the orientation of the orbitals. It is represented as
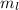 . The value of this quantum number ranges from
. The value of this quantum number ranges from
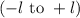 . When l = 2, the value of
. When l = 2, the value of
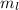 will be -2, -1, 0, +1, +2.
will be -2, -1, 0, +1, +2.
Mass number is defined as the sum of number of protons and neutrons that are present in an atom. It is represented by A.
Azimuthal or angular momentum Quantum Number : It describes the shape of the orbital. It is represented as 'l'. The value of l ranges from 0 to (n-1). For l = 0,1,2,3... the orbitals are s, p, d, f...
Principle Quantum Number : It describes the size of the orbital and the main energy level of an electron in an atom. It is represented by n. Where, n = 1,2,3,4....
Atomic number is defined as the number of protons or number of electrons that are present in an atom. It is represented by Z.