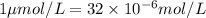Answer:
FOR H_2
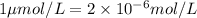
Explanation:
Given data:
concentration
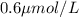
WE KNOW THAT
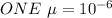
therefore
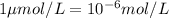
Now for conversion of mol to gram need the molar mass of element in which particular value convert to.
let us take

we know that molar mass of
 is 2 gram
is 2 gram
therefore
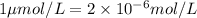
let take other example, for

we know that molar mass of O_2 is 32 gram
therefore