Answer:
When n is gamma:
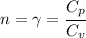
Also known as the heat capacity ratio.
Step-by-step explanation:
For an ideal gas of constant heat capacity, if we want an adiabatic process, that is, a process in which there is no entropy change, we will have the following relation:
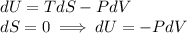
But we also know that
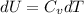
Moreover, we have by the ideal gas law:
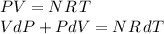
Now, if we eliminate dT from the last equations, we get:
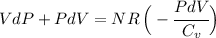
If we act algebraically on this, we get:
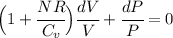
Now, the coeffcient on the left :
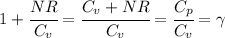
Where we have made use of so called Mayer's relation for an ideal gas:
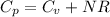
Going back to our last equation, we find:
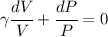
We can integrate this and get:
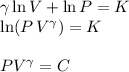
Where K and C are just integration constants.