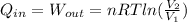Answer:
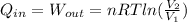
Step-by-step explanation:
According to the first thermodynamic law, the energy must be conserved so:
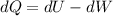
Where Q is the heat transmitted to the system, U is the internal energy and W is the work done by the system.
This equation can be solved by integration between an initial and a final state:
(1)
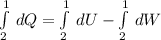
As per work definition:
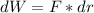
For pressure the force F equials the pressure multiplied by the area of the piston, and considering dx as the displacement:
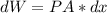
Here A*dx equals the differential volume of the piston, and considering that any increment in volume is a work done by the system, the sign is negative, so:
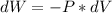
So the third integral in equation (1) is:
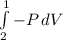
Considering the gas as ideal, the pressure can be calculated as
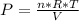 , so:
, so:
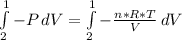
In this particular case as the systems is closed and the temperature constant, n, R and T are constants:
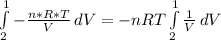
Replacion this and solving equation (1) between state 1 and 2:
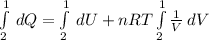
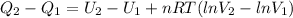
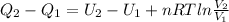
The internal energy depends only on the temperature of the gas, so there is no internal energy change
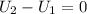 , so the heat exchanged to the system equals the work done by the system:
, so the heat exchanged to the system equals the work done by the system: