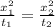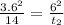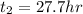Answer:
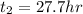
Step-by-step explanation:
Given data:
carbon concentration = 0.54%
from the relation given below calculate the time required to achieve concentration at 6.00 mm from surface
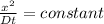
D considered constant
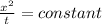
here, x POSITION FROM SURFACE, t is time required to achieve concentration