Answer:
specific volume by ideal gas equation = 0.01917 m³/kg
specific volume by compressibility chart = 0.01246 m³/kg
specif volume by super heated stream table is 0.0114810 m³/kg
Step-by-step explanation:
given data
temperature T = 350°C = 623 K
pressure P = 15 MPa = 15000 kPa
to find out
specific volume by ideal-gas equation ,generalized compressibility chart and steam tables
solution
we will apply here ideal gas equation that is
specific volume =
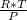 ..............1
..............1
here P is pressure and T is temperature and R is gas constant i.e 0.4615 kJ/kg-K
specific volume =
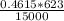
specific volume = 0.01917 m³/kg
and
by the compressibility chart
critical pressure of water Pcr = 22.06 Mpa
and critical temperature of water Tcr = 647.1 K
so
reduced pressure will be =
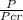
reduced pressure =
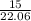 = 0.68 Mpa
= 0.68 Mpa
and
reduced temperature will be =
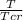
reduced pressure =
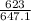 = 0.963 K
= 0.963 K
so by compressibility chart pressure 0.68 Mpa and temperature 0.963 K
compressibility factor Z is 0.65
so
specific volume = compressibility factor Z × ideal specific volume
specific volume = 0.65 × 0.01917
specific volume = 0.01246 m³/kg
and
by the steam table
use here super heated stream table for
pressure = 15 Mpa
ans temperature = 350°C
so
specif volume by super heated stream table is 0.0114810 m³/kg