Answer:
Z= 0.868
Step-by-step explanation:
Given that
P= 3 MPa
T = 160 K
We know that
P v= Z R T
P= Pressure
v = specific volume
R= gas constant
T = Absolute temperature
Z= Compressibility factor
Here specific volume of gas is not given so we assume that specific volume gas
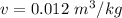
We know that for oxygen gas constant
R = 0.259 KJ/kg.K
Now by putting the values
P v = Z R T
3000 x 0.012 = Z x 0.259 x 160
Z= 0.868
So Compressibility factor is 0.868.