Answer:
The amount of carbon is the sample is 0.163 grams.
Step-by-step explanation:
Mass of carbon dioxide produced by the sample = 0.600 g
Moles of carbon dioxide =
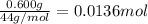
Moles of carbon atom in 0.01364 moles of carbon dioxide:
1 × 0.0136 mol = 0.0136 mol
Mass of 0.01364 moles of carbon :
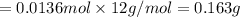
The amount of carbon is the sample is 0.163 grams.