Answer: 232 nm
Explanation: As it is well known the energy for the one dimentional box is given by:
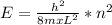
where h is the Planck constant, m the electron mass and L the width of the box. n is the energy level
For n=3 we a energy equal :9.62 * 10^-19 J= 6.01 eV
For n=1 the energy is: 1.069 *10^-19 J=0.66 eV
The energy difference Is 5.35 eV so by using this relationship
λ=1240/Energy = 232 nm